FDA Approves First CRISPR Treatment in U.S.
It was only 11 years ago that scientists Jennifer Doudna and Emmanuelle Charpentier first described a new way to edit genes, called CRISPR, in a scientific paper. The discovery is so game-changing that the pair earned the Nobel Prize in Chemistry in 2020 for how it could transform the way genetic diseases are treated. Now, on Dec. 8, the U.S. Food and Drug Administration (FDA) approved the very first treatment in the country based on the technology.
In the medical world, that’s lightning speed. “It’s incredible,” says Doudna, professor of chemistry and molecular and cell biology at the University of California, Berkeley. “It’s so exciting to see how fast, and frankly how safely and effectively, this therapy is being rolled out in humans.”
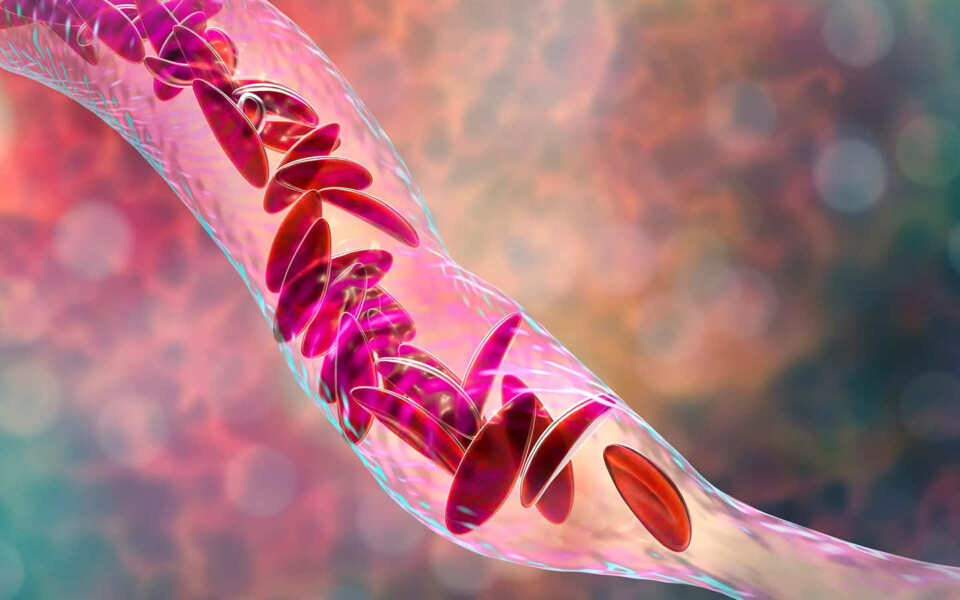
The U.K. had already approved the CRISPR treatment, called exa-cel (brand name: Casgevy), from Vertex Pharmaceuticals and CRISPR Therapeutics (which was co-founded by Charpentier), to treat people with sickle cell disease and beta thalassemia. People with these conditions are born with genetically abnormal blood cells. In the case of sickle cell, the mutations in the gene coding for hemoglobin can cause blood cells to form a sickle shape, rather than a spherical one, and clog up small blood vessels, leading to potentially life-threatening episodes of pain and a higher risk of stroke. Patients with beta thalassemia develop anemia so severe that it can damage organs. Both conditions require lifelong and repeated blood transfusions. CRISPR can increase the population of healthy blood cells in both groups of patients. The FDA approved exa-cel for sickle cell disease, and will make a decision about treating beta thalassemia by March 2024.
The agency also approved another more traditional gene therapy for the disease as well: lovo-cel (brand name: Lyfgenia) from bluebird bio, giving sickle cell patients two powerful new ways of controlling the debilitating and painful attacks that are the hallmark of their disease.